
- September 2, 2025
- Top Search
- 9:36 am
“🎧 Listen to this article instead of reading (audio version below).”
Atrial fibrillation (AF) affects millions globally and remains a leading cause of stroke. Traditionally, doctors rely on general risk scores like CHA₂DS₂-VASc and HAS-BLED to decide whether a patient needs anticoagulants—known as blood thinners. However, these tools offer averages rather than personalized guidance. In August 2025, researchers at Mount Sinai’s Icahn School of Medicine introduced a groundbreaking AI model that may shift this paradigm by delivering patient-specific recommendations.
Why This AI Model Matters
AF occurs when the heart’s upper chambers quiver instead of beating normally, allowing blood clots to form and potentially cause strokes. Standard treatment involves prescribing blood thinners to reduce clot risk—but these medications can increase the chance of serious bleeding.
Mount Sinai researchers have developed an AI model that analyzes a patient’s complete electronic health record to predict both stroke and bleeding risk. Based on that data, the model recommends whether anticoagulation is beneficial for that individual—going beyond clinical averages.
How the AI Works
This innovative model aggregates a vast range of data—1.8 million patient records, covering over 21 million doctor visits, 82 million notes, and 1.2 billion data points—to train a system tailored to individual risk profiles.
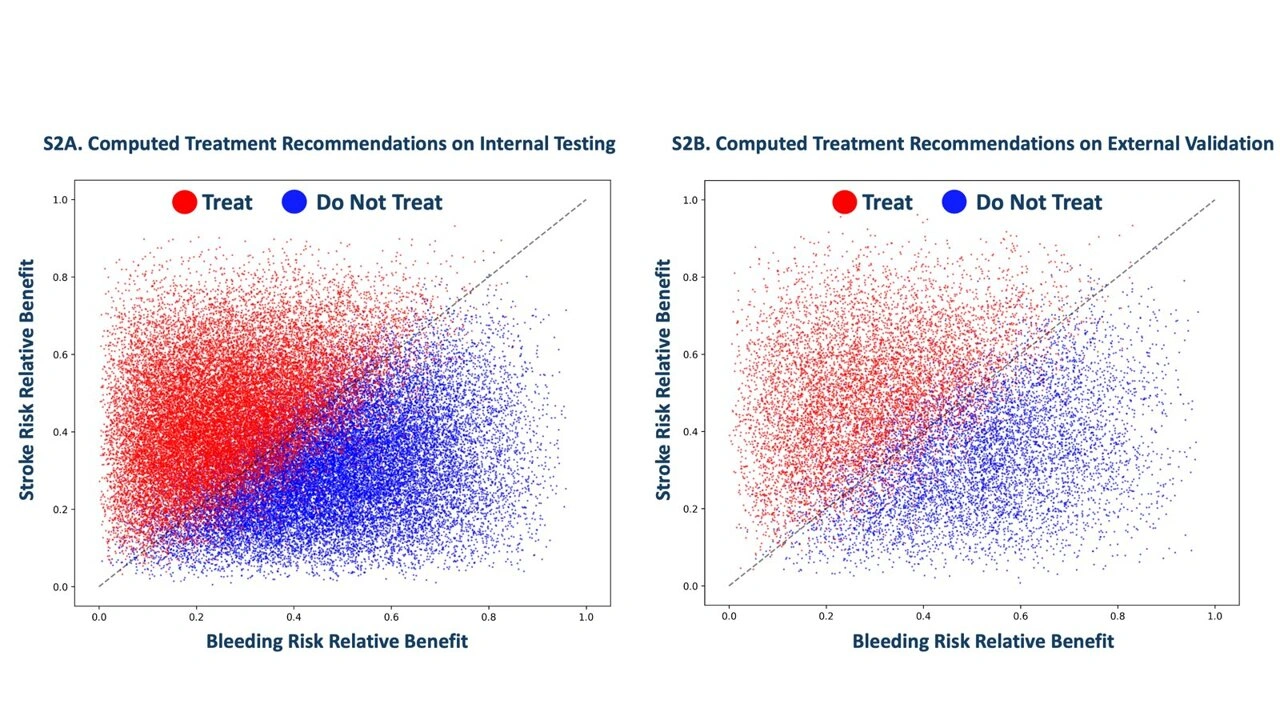
During validation, the model was tested on nearly 39,000 AF patients within Mount Sinai and externally on almost 13,000 patients using Stanford-based datasets. Its recommendations showed strong alignment with actual outcomes—helping to minimize stroke and bleeding risks. Notably, about half of the patients who would have gotten blood thinners under current guidelines were instead advised to forgo them.
What Sets It Apart?
Current clinical scores—like CHA₂DS₂-VASc for stroke and HAS-BLED for bleeding—evaluate broad risk trends, not individual profiles. In contrast, this AI system evaluates an individual’s actual clinical features to deliver a net-benefit recommendation customized for that person. It’s the first known AI tool designed with this level of individualized, dual-risk assessment for AF patients.
Why This Could Be a Game-Changer
Better Outcomes: Patients less likely to benefit from blood thinners could safely avoid them, reducing bleeding risk.
Precision Medicine: Treatment becomes tailored to individual needs rather than broad guideline-based standards.
Healthcare Efficiency: Fewer unnecessary prescriptions could mean lower costs and fewer treatment complications.
This model could revolutionize how AF patients are managed—shifting treatment decisions from general rules to personalized, data-driven strategies.
Looking Ahead: Considerations and Next Steps
Real-World Use: While results are promising, larger clinical trials are needed to ensure safety across diverse populations.
Integration: Embedding this system into everyday clinical workflows will require thoughtful implementation.
Transparency: As with many AI tools, interpretability and clinician trust are key to acceptance.
Ethics and Bias: Ensuring that the model performs fairly and equitably across different patient groups is essential.
FAQs
It leverages a patient’s entire medical record—including comorbidities, notes, visits—to recommend anticoagulation based on personalized stroke vs. bleeding risk, rather than average population data.
The AI reclassified nearly 50% of AF patients—who would normally receive anticoagulants under current guidelines—as not needing them.
The model was trained on 1.8 million patients’ electronic health record data, covering over 21 million doctor visits, 82 million notes, and 1.2 billion data points.
Yes—tested on almost 39,000 Mount Sinai patients and externally on nearly 13,000 Stanford-derived cases, showing consistent performance.
While highly promising, widespread clinical adoption requires further validation studies, transparency on how recommendations are derived, and integration into clinical systems.

Recent Posts:


AI Chatbot 2025: From ELIZA to Next-Gen Smart Assistants


